HemCon® GuardaCare XR Surgical
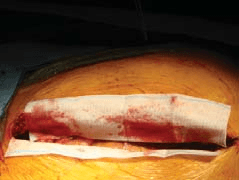
Positive Outcomes. STAT.
SURGICAL HEMOSTAT FOR TEMPORARY CONTROL OF BLEEDING IN THE SURGICAL SUITE
HemCon GuardaCare XR Surgical is an x-ray detectable surgical hemostat ideally suited for temporary control of minor to severe bleeding of surgical wounds and traumatic injuries.
• Cost effective:
Lower cost than other alternatives; minimizes lap pad and sponge utilization. Rapid bleeding control can result in less need for cauterization.
• Fast hemostasis:
Achieves hemostasis quickly even on severe bleeding1,3; dressing minimizes blood loss4,6. Reduced blood loss can reduce the need for transfusions.
• Dependable:
Works with low to severe bleeding; provides localized support of clotting; reduces visual obstruction to the surgical field; non-shedding.
• Easy to use:
Reduces direct pressure protocol to free up staff; familiar configuration conforms to a variety of wound and anatomy types; no prep time before use.
• Safe:
No human or bovine thrombin; works independently of the clotting cascade; contains radio-opaque element to easily retrieve all dressings which may help avoid adverse surgical incidents; double pouched for aseptic surgical field transfer.
INDICATION FOR USE
HemCon GuardaCare XR Surgical is intended for use as a hemostatic dressing for temporary control of severely bleeding wounds such as surgical wounds and traumatic injuries.
HOW HEMCON BANDAGES WORK
GuardaCareXR Surgical is a chitosan impregnated gauze with an x-ray detectable element. Chitosan is a naturally occurring, bio-compatible polysaccharide and the hemostatic properties of chitosan enhance the ability of the medical gauze to control bleeding. The robust uniformly applied chitosan coating on the gauze allows for optimal chitosan blood interaction to effectively control bleeding. The chitosan further reduces blood loss by helping the dressing conform to the wound site while providing a physical barrier to prevent bleeding. This mechanism of action supports localized clotting with and on the gauze to stop bleeding quickly and independently of the clotting cascade. The dressing readily conforms to wound surfaces with complex geometries to allow efficient stanching of all bleeding.
APPLICATION GUIDE
- Place or pack dressing completely over the source of bleeding. Make sure to contact all bleeding surfaces.
- Apply pressure until bleeding is controlled. More than one dressing may be required to control bleeding.
REMOVAL INSTRUCTIONS
- Remove dressing within 24 hours gently pulling on the edge of the dressing. If dressing is difficult to remove, wet with normal saline solution and attempt removal.
- If bleeding resumes after careful removal of the dressing, the use of standard surgical techniques may be required to adequately repair the source of bleeding.
- Rinse/flush wound site as part of your standard procedure protocol.
- Do not leave dressing within the body cavity. Dressing needs to be removed within 24 hours. Do not re-use or resterilize the dressing.
ADDITIONAL NOTES
- Show product removal directions on package to medical personnel if patient is transported and dressing remains in place.
- If cutting the dressing to a smaller desired size, be sure to include the radioopaque element in the parts you are planning to use. Discard any unused portion of the product.

CLINICAL AND IN-VIVO EFFICACY
GuardaCareXR Surgical was tested in different bleeding models to evaluate efficacy versus control sponges, gazes and coated gauze (competitive). GuardaCareXR Surgical exhibited positive results when compared to the control sponges and/or gazes in the test models.
| Study | Author | Publication |
|---|---|---|
| GuardaCare®XR Surgical Following Posterior Repair in Management Of Uterine Rupturing To The Multiple Vaginal Lacerations | Baron, J.D., et al. | Presented at Soroka Medical Center OBGYN Congress, Israel, January, 2012 |
| Hemostatic During Multiple Flap Face Lift Procedure with GuardaCare®XR Surgical | Case Study | |
| Hemostasis with GuardaCare®XR Surgical in a Reduction Mammoplasty (Breast Reduction Procedure) | Courtesy of Dr. Bianco Rosenberg-Hagen, Israel | Case Study |
| Use of GuardaCare®XR Surgical During Coronary Artery Bypass Graft (CABG) Surgery | Reza Khaffafi, MD, North Hills Hospital, TX | Case Study |
| Hemostatic Surfactance Of GuardaCareXR Surgical In Two In Vivo Surgical Injury Models In Swine | Xue, Hua, MD; et al. | Data presented at the 71st Annual Meeting of the American Association for the Surgery of Trauma (AAST), Sept. 12-15, 2012 - Kotel, HI |
| Comparison of Hemostatic Efficacy of ChitoGauze and Control Gauze in a Swine Femoral Arterial Injury Model | Xue, Hua, MD; et al. | Presented at Advanced Technology Applications for Combat Casualty Care (ATACCC) 2009 |
| Comparison of ChitoGauze and Combat Gauze for Hemorrhage Control in a Swine Model | Schwartz, Richard et al. | Published in Hospital Emergency Care 2011; 15:477-482 |
| Comparison of Novel Hemostatic Gazes to QuickClot Combat Gauze in a Standardized Swine Model of Uncontrolled Hemorrhage | Jason M. Roll, PhD, et al. | Published in Journal of Trauma Acute Care Surg 2013 Aug; 75 (2 Suppl 2):S150-6 |
ORDER INFORMATION
| Product Name | Part Number | Configuration |
|---|---|---|
| GuardaCare XR Surgical, 2in x 2in, 8 ply (12in x 16in) | 1031 | 10/bx, 100/cs |
| GuardaCare XR Surgical, 4in x 4in, 8 ply (4in x 32in) | 1032 | 10/bx, 100/cs |
| GuardaCare XR Surgical, 4in x 2 yd | 1033 | 5/bx, 50/cs |
FDA 510(K): K103641
Tax ID: 81-2091181
MMF-230 Rev 3 / 10/16
