OneStopTM Vascular
Safe and effective hemostatic device for vascular closure procedures
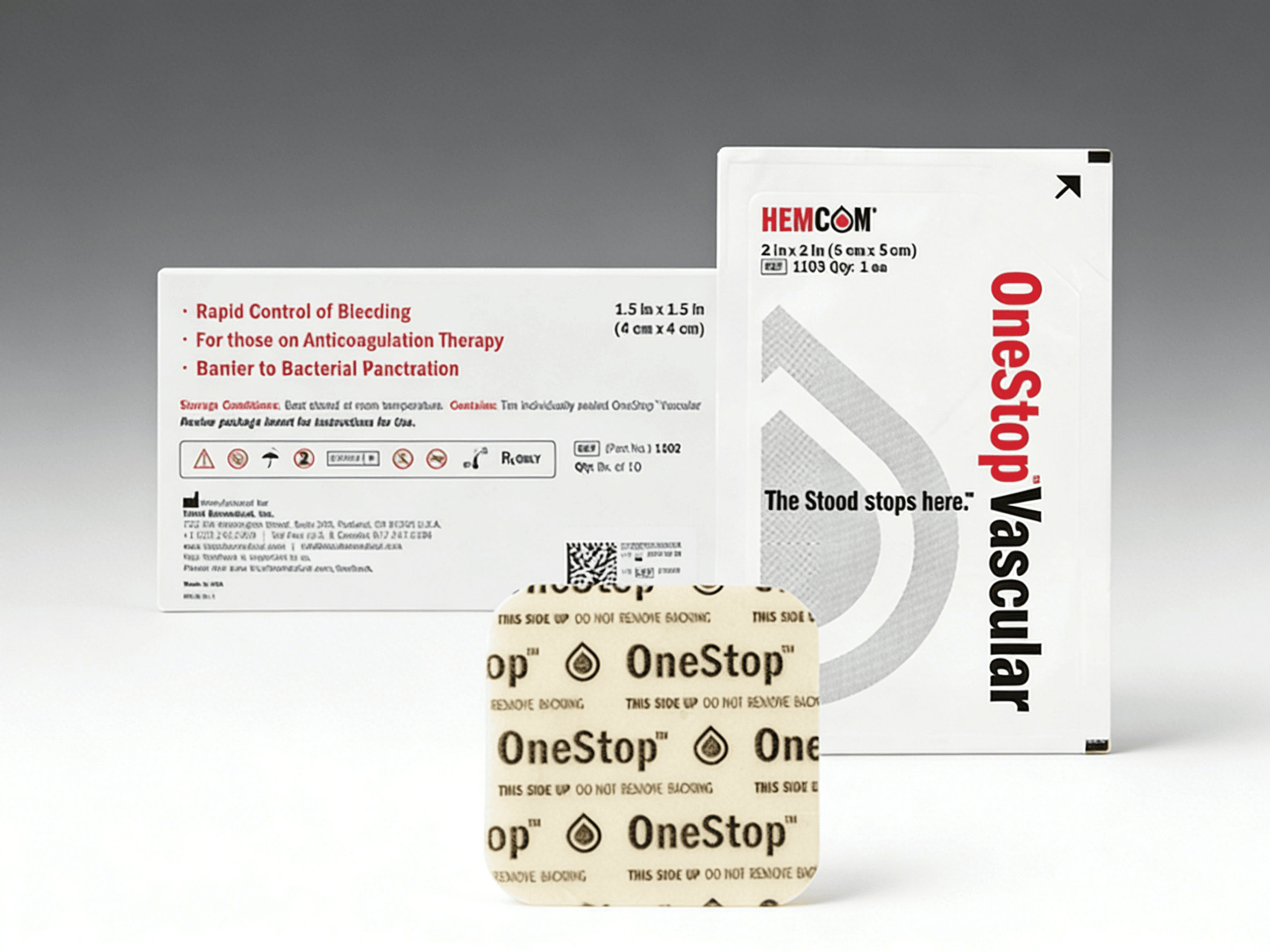
Safe and effective hemostatic device
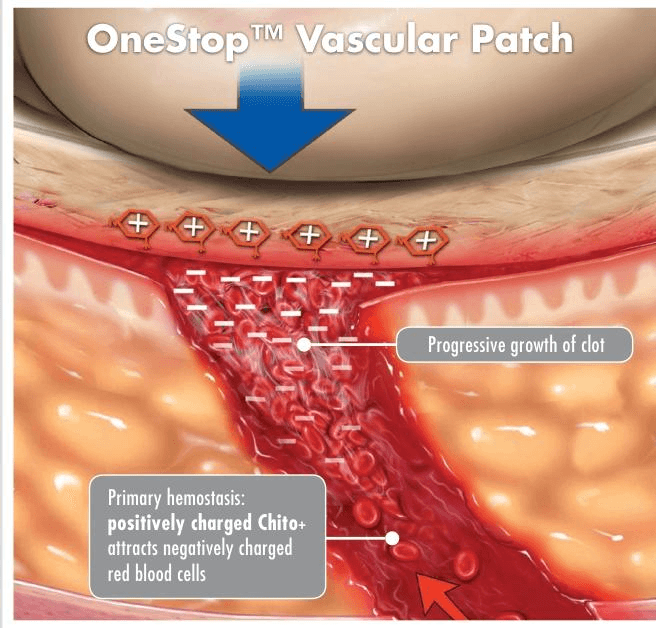
Tricol's unique chitosan technology, Chito+, works independently of the body's clotting cascade and forms a strong, supportive, adhesive seal when in contact with blood.
An ideal solution for safe bleeding control
• Rapidly controls bleeding
• Allows hemostasis in patients on anticoagulation therapy
• Provides an antibacterial barrier to MRSA, VRE, and many others*
• Maintains structural integrity — won't crack, crumble, shed, or become saturated
• Clinically proven to reduce hold times
• Can be used as an adjunct with mechanical closure devices
INDICATIONS FOR USE
The OneStop™ Vascular Patch is intended for local management of bleeding wounds and to provide a barrier to bacterial penetration of the dressing in all patients. It promotes rapid control of bleeding (hemostasis) in patients, including those on anticoagulation therapy. The dressing is indicated for the following wounds: vascular procedure sites and sites involving percutaneous catheters, tubes, and pins.
UNIQUE CHITOSAN-BASED BIOTECHNOLOGY
OneStop™ products are composed of chitosan, a naturally occurring, biocompatible polysaccharide derived from shrimp shells. Tricol's proprietary poly is a unique muco-adhesive formulation that quickly works outside the body's own clotting cascade.
The positive molecular charge of Chito+ attracts negatively charged red blood cells, similar to a magnet. As the red blood cells are drawn to the bandage, a clot is formed over the wound. The result:
- A tight seal over the dermal wound site
- Fast hemostasis — separate from, and supportive of, the body's natural ability to clot
- An antibacterial barrier*
SPECIFICATIONS
- Non-invasive hemostatic patch
- 1.5" x 1.5" (4cm x 4cm)
- 2" x 2" (5cm x 5cm)
- Latex-free
EASY APPLICATION
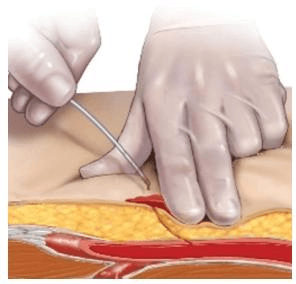
- Allow nickel-sized (~ 2 cm) drop of blood to form at puncture site. Blood is required for dressing to adhere. (Do not cleanse puncture site or moisten with saline solution.)
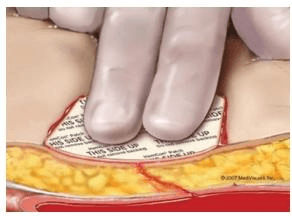
- With printed side facing up, place dressing directly on puncture site. Dressing can be cut to size. Do not remove the backing.
- Hold digital pressure until bleeding is controlled.
- After bleeding has stopped, secure OneStop™ Vascular with appropriate dressing (not included).
EASY REMOVAL
- Recheck the wound for potential bleeding as necessary. If hemostasis is not achieved or for recurrent bleeding, remove dressing with saline or water and re-apply a new dressing until hemostasis is achieved.
- Remove dressing within 48 hours by irrigating with saline or water while gently pulling up on the corner of the dressing.
ORDERING INFORMATION
| Item Number | Description | Packaging |
|---|---|---|
| 1102 | 1.5" x 1.5" (4cm x 4cm) | 10/box, 100/case |
| 1103 | 2" x 2" (5cm x 5cm) | 10/box, 100/case |
FDA 510(k) K150916
Tax ID 81-2091181
CLINICAL EVIDENCE
- Oozawa S, et al. "A New Hemostasis Tool after Percutaneous Angioplasty: The HemCon® Pad Hemostasis Device." J Vasc Med Surg 2014; 1:125.
- Mat Nor K, et al. "Achieving Haemostasis of Femoral Artery Puncture Post Angiographic Procedures by Manual Compression. A Comparison Study Between Gauze Pad and HemCon Pad." ECR2013.
- Arbel MD, et al. "Usage of Chitosan for Femoral (USF) Haemostasis after Percutaneous Procedures: Comparative Open Label Study." Eurointervention 2010; Apr; 6 (a9):1104-9.
- Kranokpiraksa P, et al. "Hemostatic Efficacy of Chitosan-based Bandage for Closure of Percutaneous Arterial Access Sites: An Experimental Study in Heparinized Sheep Model." (Oregon Health & Sciences University). 2009.
- HemCon Patch PRO Suggested Protocol. (MMF-185) (Tricol Biomedical). 2014.
- Cath Lab Case Study of HemCon Bandages (St. Elizabeth Medical Center). 2008.
- Post-Procedural Ambulation Guidelines Following Catheterization (MMF-151) (Tricol Biomedical). 2009.
